Team Uses AI and Robotics to Formulate Therapeutic Proteins
Stabilized enzyme could offer new hope to those with spinal cord injuries
By employing artificial intelligence (AI) and robotics a team led by Rutgers School of Engineering researchers successfully stabilized an enzyme able to degrade scar tissue resulting from spinal cord injuries and promote tissue regeneration.
The study, (https://onlinelibrary.wiley.com/doi/full/10.1002/adhm.202102101), published in Advanced Healthcare Materials, details the team’s ground-breaking stabilization of the enzyme Chondroitinase ABC (ChABC) for treatment of spinal cord injuries.
“I’m excited by this study for multiple reasons,” said Adam Gormley, the project’s principal investigator and a Rutgers School of Engineering (SoE) assistant professor of biomedical engineering. “First, it’s one of the first times artificial intelligence and robotics have been used to formulate highly sensitive therapeutic proteins and extend their activity by such a large amount. It’s a major scientific achievement.”
Gormley’s research is also motivated, in part, by a personal connection to spinal cord injury. “I’ll never forget being at the hospital and learning a close college friend would likely never walk again after being paralyzed from the waist down after a mountain biking accident,” he recalled.
“The therapy we are developing may someday help people such as my friend lessen the scar on their spinal cords and regain function,” Gormley said. “This is a great reason to wake up in the morning and fight to further the science and potential therapy.”
First, it’s one of the first times artificial intelligence and robotics have been used to formulate highly sensitive therapeutic proteins and extend their activity by such a large amount. It’s a major scientific achievement.
Adam Gormley
BME Assistant Professor
A Collaborative, Funded Project
Project research, according to SoE biomedical engineering doctoral student and study lead author Shashank Kosuri, was supported by grants from the National Institutes of Health, the National Science Foundation, and The New Jersey Commission on Spinal Cord research.
The Rutgers team also included Department of Biomedical Engineering professor Li Cai and distinguished professor Martin Yarmush as well as several SoE-affiliated students. In addition, Michael Webb, an assistant professor in Princeton University’s Department of Chemical and Biological Engineering, and graduate students in his lab collaborated on the project.
According to Kosuri, spinal cord injuries, or SCIs, can negatively impact the physical, psychological, and socio-economic well-being of patients and their families. Soon after an SCI, a secondary cascade of inflammation produces a dense scar tissue that can inhibit or prevent nervous tissue regeneration.
Kosuri explained that the enzyme ChABC is known to degrade scar tissue molecules and promote tissue regeneration, yet it is highly unstable at the human body temperature of 98.6° F. and loses all activity within a few hours. “This necessitates multiple, expensive infusions at very high doses to maintain therapeutic efficacy.”
Stabilizing a Therapeutic Enzyme
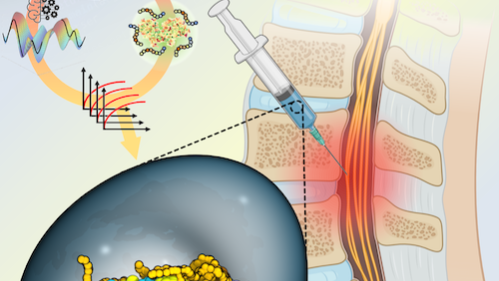
Synthetic copolymers are able to wrap around enzymes such as ChABC and stabilize them in hostile microenvironments. The researchers, according to Kosuri, coupled an AI-driven approach with liquid handling robotics to synthesize and test the ability of numerous copolymers to stabilize ChABC and maintain its activity at 98.6° F.
While the researchers were able to identify several copolymers that performed well, Kosuri reported that, “Remarkably, one copolymer combination even continued to retain 30% of the enzyme for over a week.”
The study’s promising results potentially offer new hope for patients with spinal cord injuries.
“We’re currently investigating their efficacy with mice in an in vivo contusion injury model that is clinically relevant to human spinal cord injuries,” Kosuri said. “ And we have already submitted the provisional patent for the top polymer candidates that stabilized ChABC.”